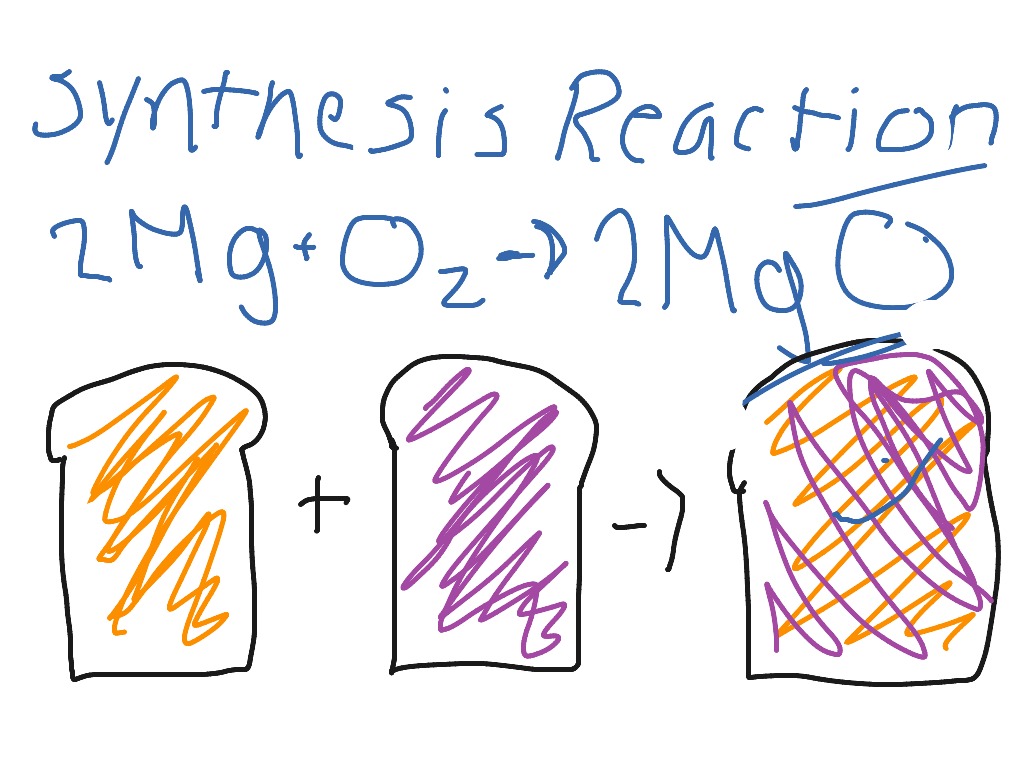Synthesis reactions are a fundamental concept in organic chemistry, involving the combination of two or more molecules to form a new compound. These reactions are crucial in the production of various chemicals, pharmaceuticals, and materials. To illustrate the principles of synthesis reactions, let's consider a classic example: the synthesis of ammonia (NH3) from nitrogen (N2) and hydrogen (H2). This reaction is not only a straightforward demonstration of synthesis but also an industrially significant process, known as the Haber-Bosch process.
Introduction to Synthesis Reactions

Synthesis reactions are characterized by the formation of a new chemical bond between the reactants, resulting in a product with a unique set of physical and chemical properties. The general equation for a synthesis reaction can be represented as A + B → AB, where A and B are the reactants, and AB is the product. Synthesis reactions can involve the combination of elements or compounds, and they are often accompanied by the release or absorption of energy. In the case of the Haber-Bosch process, the synthesis of ammonia from nitrogen and hydrogen is an exothermic reaction, meaning it releases energy.
Key Points
- Synthesis reactions involve the combination of molecules to form a new compound.
- The Haber-Bosch process is a classic example of a synthesis reaction, producing ammonia from nitrogen and hydrogen.
- Synthesis reactions can be exothermic or endothermic, depending on the energy change.
- Understanding synthesis reactions is crucial for the production of various chemicals and materials.
- The conditions under which synthesis reactions occur, such as temperature and pressure, can significantly affect the yield and rate of the reaction.
The Haber-Bosch Process: A Detailed Example
The Haber-Bosch process is a complex synthesis reaction that requires specific conditions to achieve a high yield of ammonia. The reaction equation is N2 + 3H2 → 2NH3. This process involves the use of an iron catalyst under high pressure (around 200 atmospheres) and temperature (approximately 450°C). The catalyst plays a crucial role in lowering the activation energy required for the reaction to occur, thus increasing the reaction rate and efficiency. The Haber-Bosch process is not only an excellent example of a synthesis reaction but also a testament to the importance of catalysts in chemical reactions.
| Reactants | Conditions | Product |
|---|---|---|
| Nitrogen (N2) and Hydrogen (H2) | High pressure, high temperature, iron catalyst | Ammonia (NH3) |

Importance of Synthesis Reactions

Synthesis reactions are foundational in various industries, including pharmaceuticals, agrochemicals, and materials science. They enable the creation of new compounds with tailored properties, which can address specific needs or challenges. For instance, the synthesis of polymers from monomers has led to the development of plastics, fibers, and other materials that are integral to modern society. Similarly, the synthesis of complex molecules is crucial in the development of new drugs, where the structure of the molecule directly influences its efficacy and safety.
Challenges and Future Directions
Despite the advancements in synthesis reactions, there are ongoing challenges, particularly in terms of efficiency, sustainability, and the development of new catalysts. The quest for greener synthesis methods, which minimize waste and reduce environmental impact, is a significant focus of current research. Additionally, the use of computational tools and machine learning algorithms to predict and optimize synthesis conditions is an emerging area, promising to revolutionize the field by making synthesis reactions more predictable and efficient.
In conclusion, synthesis reactions, as exemplified by the Haber-Bosch process, are a cornerstone of chemistry, underpinning the production of a vast array of chemicals and materials. Understanding the principles of synthesis reactions, including the role of catalysts, reaction conditions, and the importance of energy changes, is essential for advancing various fields of science and technology. As research continues to evolve, the development of more sustainable, efficient, and innovative synthesis methods will play a critical role in addressing global challenges and advancing societal progress.
What is a synthesis reaction, and how does it differ from other types of chemical reactions?
+A synthesis reaction involves the combination of two or more molecules to form a new compound. It differs from other reactions, such as decomposition or replacement reactions, in that it results in the formation of a new chemical bond between the reactants, leading to a product with unique properties.
What role do catalysts play in synthesis reactions, and why are they important?
+Catalysts are substances that increase the rate of a chemical reaction without being consumed in the process. In synthesis reactions, catalysts can lower the activation energy required for the reaction to occur, making the process more efficient and allowing it to proceed under milder conditions. This is crucial for industrial applications, where the cost and feasibility of a process can depend significantly on the efficiency of the catalyst used.
What are some of the challenges and future directions in the field of synthesis reactions?
+Challenges in synthesis reactions include the development of more sustainable and efficient methods, the design of new catalysts, and the prediction and optimization of reaction conditions. Future directions involve the integration of computational tools and machine learning algorithms to predict reaction outcomes and the exploration of greener synthesis methods that minimize waste and environmental impact.