The percent recovery formula is a fundamental concept in analytical chemistry, used to determine the efficiency of a separation or extraction process. It is a measure of the amount of a substance that is recovered from a sample, compared to the initial amount present. Understanding the percent recovery formula is crucial in various fields, including pharmaceuticals, environmental monitoring, and food safety. In this article, we will delve into the details of the percent recovery formula, its significance, and its applications.
Definition and Formula

The percent recovery, also known as the percentage recovery or recovery rate, is calculated using the following formula:
Percent Recovery (%) = (Amount Recovered / Amount Initially Present) x 100
This formula is straightforward, yet it provides valuable insights into the effectiveness of a separation or extraction process. The amount recovered refers to the quantity of the substance that is successfully isolated or extracted from the sample, while the amount initially present represents the original quantity of the substance in the sample.
Significance of Percent Recovery
The percent recovery formula is essential in evaluating the performance of various analytical techniques, such as chromatography, spectroscopy, and extraction methods. A high percent recovery indicates that the method is efficient and effective in isolating the desired substance, while a low percent recovery suggests that the method may be flawed or that there are losses during the process.
| Percent Recovery Range | Interpretation |
|---|---|
| 90-100% | Excellent recovery, method is efficient and effective |
| 80-89% | Good recovery, method is satisfactory but may require optimization |
| 70-79% | Fair recovery, method may require significant optimization or revision |
| Below 70% | Poor recovery, method is ineffective and requires substantial revision |
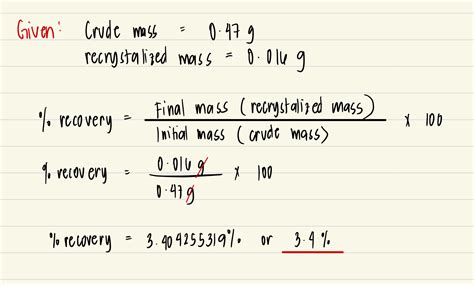
Applications of Percent Recovery

The percent recovery formula has numerous applications in various fields, including:
- Pharmaceuticals: to evaluate the purity and yield of active pharmaceutical ingredients
- Environmental monitoring: to assess the efficiency of methods for extracting pollutants from environmental samples
- Food safety: to determine the effectiveness of methods for detecting and quantifying contaminants in food products
- Biotechnology: to optimize the recovery of biomolecules, such as proteins and nucleic acids, from complex samples
Calculating Percent Recovery
To illustrate the calculation of percent recovery, let’s consider a simple example. Suppose we have a sample containing 100 mg of a target substance, and after applying an extraction method, we recover 85 mg of the substance. Using the percent recovery formula, we can calculate the percent recovery as follows:
Percent Recovery (%) = (85 mg / 100 mg) x 100 = 85%
This result indicates that the extraction method has a good recovery rate, but there may be some losses during the process.
Key Points
- The percent recovery formula is used to evaluate the efficiency of a separation or extraction process.
- A high percent recovery indicates that the method is efficient and effective.
- The percent recovery formula has numerous applications in various fields, including pharmaceuticals, environmental monitoring, and food safety.
- Calculating percent recovery involves dividing the amount recovered by the amount initially present and multiplying by 100.
- A thorough understanding of the percent recovery formula and its significance is crucial for developing and optimizing analytical methods.
Conclusion
In conclusion, the percent recovery formula is a fundamental concept in analytical chemistry, providing valuable insights into the effectiveness of separation and extraction processes. By understanding the significance and applications of the percent recovery formula, analytical chemists can develop and optimize methods that are efficient, effective, and reliable. Whether in pharmaceuticals, environmental monitoring, or food safety, the percent recovery formula is an essential tool for ensuring the quality and accuracy of analytical results.
What is the purpose of calculating percent recovery?
+The purpose of calculating percent recovery is to evaluate the efficiency of a separation or extraction process and to identify potential losses or issues during the process.
How is percent recovery calculated?
+Percent recovery is calculated by dividing the amount recovered by the amount initially present and multiplying by 100.
What are the applications of percent recovery?
+The percent recovery formula has numerous applications in various fields, including pharmaceuticals, environmental monitoring, food safety, and biotechnology.



